|
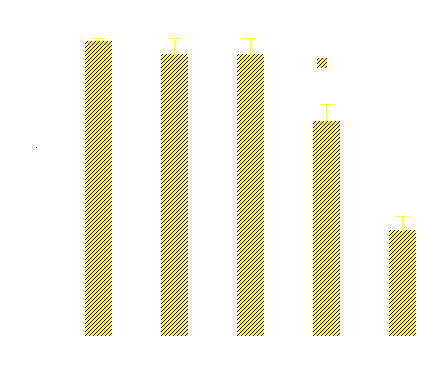
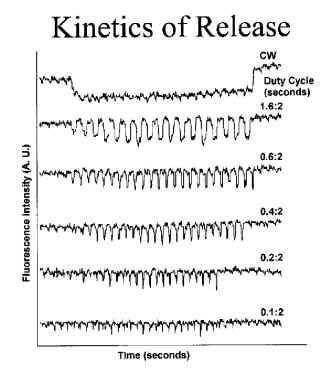
Summary of Research In our laboratory, we observed that drug encapsulation in polymeric micelles reduced the intracellular drug uptake (Figure 1, white bars) while the ultrasonic irradiation of the cells enhanced the uptake of the micellar-encapsulated drug (Figure 1, yellow bars). The ultrasonic increase of the drug uptake, at least partly, resulted from drug release from encapsulating micelles (Figure 2).
Based on these findings, we are presently developing a novel technique of controlled and targeted delivery of drugs to solid tumors. The technique involves drug encapsulation in polymeric micelles followed by controlled release at the target site triggered by focused ultrasound. The advantages of ultrasonic drug delivery and micellar drug carriers are combined into a novel technology that delivers high local concentrations of drugs to tissues exposed to localized acoustic energy. Ultrasound has many advantages as a mediator of drug delivery: the technique is non-invasive; it can be carefully controlled and focused on the target tissue; ultrasound appears to "activate," or to enhance the pharmacological activity of some drugs; it enhances drug transport through tissues and across cell membranes; and it can simultaneously create a hyperthermic condition that can enhance the destruction of cancerous tissues. Drug Resistant Tumors In our research, we have demonstrated that the ultrasonic-activated drug delivery in Pluronic micelles provided substantial decrease of IC50 of two anti-cancer drugs in drug-sensitive and Multi-Drug Resistant (MDR) cells. Fluorescence microscopy and flow cytometry of the cells indicated that this effect, at least partly, was caused by the enhanced release of the Pluronic-associated drugs from the cytoplasmic vesicles of the cells; this resulted in the enhanced Pluronic and drug accumulation in cell nuclei (Figure 3). Another important mechanism involved in the sensitization of MDR cells by Pluronic micelles may be related to the reduced intracellular energy production. We find these results very exciting since they have manifested the feasibility of treating MDR tumors using acoustically activated micellar drug delivery.
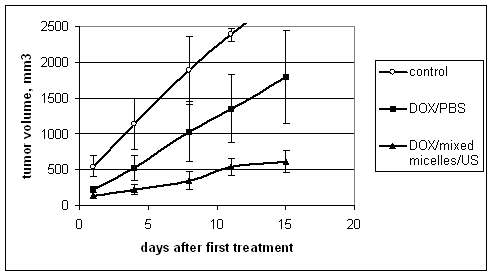
These results warrant continued study to verify in vivo feasibility of micelle/ultrasound technology using animal experiments. Animal experiments performed so far confirmed drug targeting to solid tumors, enhanced intracellular drug uptake by tumor cells, and a substantial reduction of the tumor growth rates. More animal experiments are currently under way. In
vivo studies of drug targeting to solid tumors using micelle/ultrasound
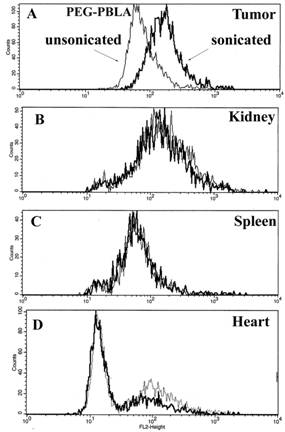
drug delivery. We tested the proposed technique of drug targeting to solid tumors in vivo using ovarian carcinoma tumor model in nu/nu mice. Drug (Doxorubicin, DOX) was encapsulated in specially designed polymeric micelles; some time after the intravenous or intratumoral drug injection, the tumor was locally irradiated by 1-MHz or 3-MHz ultrasound. We measured DOX biodistribution, tumor growth rates, and mice survival rates. The data were compared with those for a molecularly dissolved DOX, with or without tumor sonication. In separate experiments, polymeric micelles (i.e. DOX carriers) were fluorescently labeled and their biodistribution was measured. DOX and labeled micelle intracellular uptake was characterized by flow cytometry. The results of this study showed that a local ultrasonic irradiation of the tumor resulted in a substantially increased drug accumulation in the tumor cells without significant effect on the drug or drug carrier uptake by the cells of other organs indicating a substantial degree of drug targeting to the tumor cells (Figure 4).
The ultrasound effect depended on the time of ultrasound application after the drug injection; the ultrasonic enhancement of the drug internalization by the tumor cells required a preliminary passive drug accumulation in the tumor interstitium. At the experimental conditions, ultrasound did not enhance micelle extravasation. Due
to the ultrasound-enhanced drug intracellular uptake and cell killing,
the yield of the intraperitoneal (i.p.) ovarian carcinoma tumors
decreased from 70% for DOX dissolved in PBS (positive control) to 36%
for the same concentration of DOX encapsulated in Pluronic micelles
combined with a 30-s sonication of the abdominal region of a mouse (3
mg/kg DOX, i.p. injections, n ≥ 10). For
|
||||||||||||